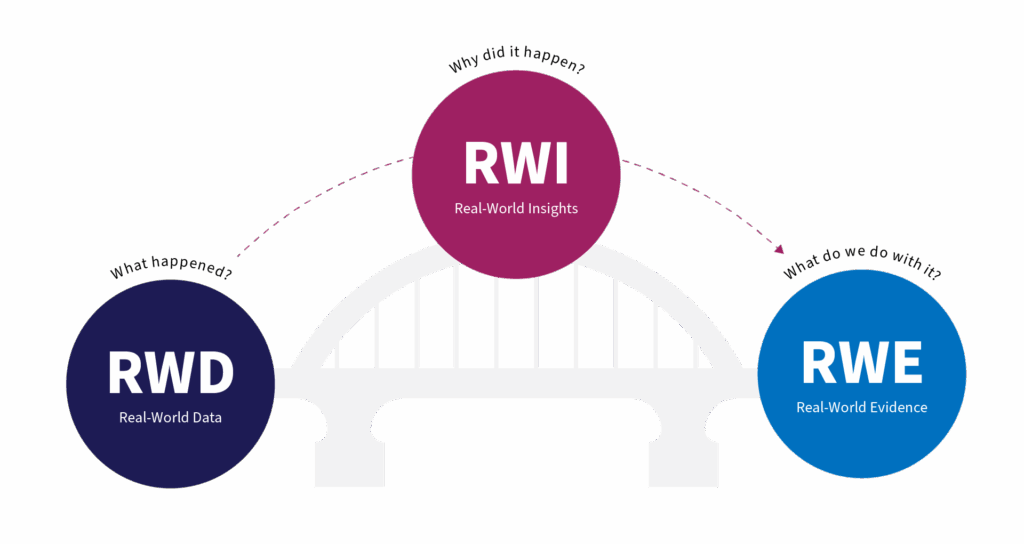
In the fast-evolving world of real-world evidence (RWE), two foundational pillars are referred to:
- Real-world Data (RWD): the raw observations from everyday clinical practice
- Real-world Evidence (RWE): the analysed outputs that support regulatory and business decisions
Real-world Insights (RWI): revealing the motivations, barriers, and decisions behind RWD that underpin real-world clinical and commercial outcomes – this approach adds the ‘why’ to the ‘what’.
This isn’t just a new term. It’s a framework that can make real-world evidence more meaningful, actionable, and human-centred. And it’s long overdue.
Understanding how these three components interact is critical for anyone working in market access, medical affairs, Health Economics Outcome Research (HEOR) , or real-world research.
What is Real-world Data (RWD)?
RWD refers to the raw, observational data captured during routine clinical practice. It is typically structured and collected passively through:
- Electronic health records (EHRs)
- Claims and billing systems
- Hospital registries
- Lab results
- Wearable health devices
- Pharmacy records
RWD answers the question: What happened?
Example: A claims dataset shows that 35% of patients with type 2 diabetes discontinued a GLP-1 therapy within three months.
That’s useful, but incomplete. We now know what happened. But why?
Introducing Real-world Insights (RWI)
RWI helps answer the “why.” It considers the qualitative and perception-based inputs that explain behaviours, decisions, and experiences not captured in structured datasets.
RWI is generated through primary market research and usually comprises of one or more of the following:
- Surveying physicians, patients, and care-givers
- In-depth interviews
- Focus groups
- Online bulletin boards
- KOL interviews
- Expert interviews
RWI answers the question: Why did it happen?
Example: In a physician survey (PMR), HCPs say they reduce dosage due to patient complaints of GI side effects.
In a patient interview study, 40% say cost and lack of reimbursement drove them to discontinue therapy.
Now we understand the behaviour behind the data.
What Is Real-World Evidence (RWE)?
RWE is the end goal—the analysed and interpreted evidence that emerges from RWD and (in some cases) RWI. It’s used to inform:
- Regulatory decisions
- Market access and reimbursement submissions
- Clinical guidelines
- HEOR modelling
- Post-marketing safety and effectiveness evaluations
RWE answers the question: What do we do with it?
It applies statistical or mixed-methods analysis to draw conclusions from real-world sources—frequently used in submissions to FDA, EMA, NICE , and other decision-making bodies.
The Relationship: RWI as the Bridge Between RWD and RWE
Without context, RWD can be descriptive but shallow. Without structure, RWI can be rich but anecdotal.
But together, they strengthen RWE.
This flow ensures that real-world evidence isn’t just statistically valid, but also strategically actionable and human-centred.
Examples for RWD, RWI, and RWE use cases
RWD
RWI
RWE
Track treatment discontinuation rates
(patient and HCP feedback)
Understand physician off-label behaviour
(interviews on rationale)
(surveys, social listening)
(insight-driven positioning)
Post-marketing safety monitoring
The Future: Integrated RWE that Includes RWI
- Payers will demand to understand not just outcomes, but drivers of patient behaviour
- Regulators are increasingly open to mixed-method approaches, especially in rare diseases and unmet needs
- Pharma and medtech companies will need richer insights to differentiate themselves in crowded or evolving markets
Final Takeaway
Real-world insights (RWI) will define the next phase of RWE and take its place as the missing piece between data and action.
When used alongside real-world data, RWI turns observations into understanding. That’s how real-world evidence is transformed into strategy.
At m360 Research, we specialise in bridging real-world data with real-world insight—helping life sciences clients go beyond dashboards and datasets to understand the behaviours, motivations, and perceptions that drive outcomes.
Through our primary market research expertise, we deliver:
- Physician and patient insights across global markets
- RWI-informed RWE strategies tailored for commercial, HEOR, and medical affairs
- Support in rare diseases, emerging markets, and early launch planning where structured data is limited
If you are keen to explore how RWI can boost your RWE contact us today at info@m360research.com

Dushyant Gupta
Chief Operation Officer


